- Anesthetize the animal with ketamine or any other rodent anesthetizer.
- Shave and wash the skin over the implantation site.
- Make an incision adjacent to the implantation site. If the implantation is planned on the backside of the animal, then the mid-scapular incision is made.
- To avoid excessive blood flow, place a hemostat onto the incision. Make an appropriate pocket for the pump by manipulating the subcutaneous tissues by hemostat. Keep in mind that the pocket should be large enough to allow the pump movement.
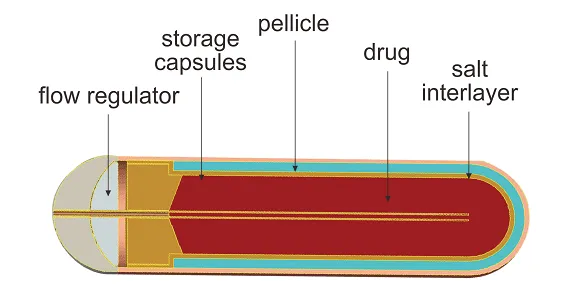
- Insert the pump containing the drug into the pocket in the incision.
- With the help of incision clips, close the wound.
The osmotic system can also be implanted in the peritoneal cavity of the larger animals. Follow the below-mentioned protocol for intraperitoneal injection:
- After anesthetizing the animal, shave and wash the skin over the implantation site.
- In the lower abdomen, make a 1 cm long midline skin incision.
- Tent the peritoneal muscles and carefully incise the peritoneal wall keeping the bowel safe.
- Insert the osmotic pump into the cavity.
- Close the abdomen with the help of sutures and incision clips.
Keep in mind that the pumps should be explanted if the animals survive after active infusion. The pumps must be removed no later than the first half-life of the test compound. The pump should be removed to measure the residual volume to confirm delivery, verify a drug’s stability, and assure the bioactivity of the test compound.










Reviews
There are no reviews yet.